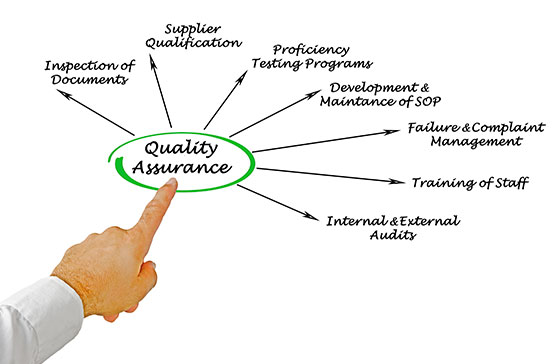
One of the prerequisites for a Licence to Manufacture Therapeutic Goods, from the TGA, is compliance with the Code of Good Manufacturing Practice for Medicinal Products. Cyclotek products are therefore manufactured under GMP conditions, and are compliant with all Commonwealth and State Regulations.
Cyclotek (Aust) Pty Ltd is a holder of a Licence to Manufacture Therapeutic Goods (No: MI-12092005-LI-000904-2) issued by the TGA.
Cyclotek Queensland Pty Ltd is a holder of a Licence to Manufacture Therapeutic Goods (No: MI-2015-LI-10055-1) issued by the TGA.
Cyclotek Melbourne Pty Ltd is a holder of a Licence to Manufacture Therapeutic Goods (No:M1-2015-LI-08410-1) issued by the TGA.
Cyclotek Pharmaceuticals Ltd (NZ) is regulated by the Ministry of Health, Office of Radiation Safety.
Cyclotek NSW Pty Ltd (Lucas Heights facility) is a holder of a Licence to Manufacture Therapeutic Goods (No: M1-2009-LI-03349-3) issued by the TGA
[contact-form-7 id=”714″ title=”Update Form”]